Neurological
Clinical Uses for Botulinum Toxin
Once perceived as the deadliest substance known to man, botulinum toxin (BT) is now known for its role in improving patient quality of life in many aspects of health care. Clinical uses for botulinum toxin-A extend far beyond cosmetic procedures, such as smoothing facial lines, that it is best known for; BT’s ability to block neuromuscular transmission is used to manage hyperhidrosis, chronic migraine, urinary incontinence, cervical dystonia, and more.
Botulinum Toxin: a History
Produced from the gram-positive bacterium Clostridium botulinum, BT is the neurotoxin responsible for botulism. While there are 8 types of BT (A, B, C1, C2, D, E, F, and G), only serotypes A and B can cause illness and have clinical applications.1 Once researchers discovered BT’s toxicity, it was considered for use as a biological weapon during World War II.2 Modern use of BT as a medical treatment began in the early 1970s when clinicians began using the type A serotype to treat strabismus.3
The FDA approved onabotulinumtoxinA for various uses including strabismus, hemifacial spasm, and blepharospasm in 1989; 13 years later, the FDA approved BT for cosmetic purposes.3
Since BT causes weakness and paralysis of the implicated muscle, it can be useful in conditions where muscle hyperactivity is the root cause.1 The neuromuscular blocking cascade caused by BT occurs in 4 steps. Step 1 includes binding to nerve cells. Step 2 is the uptake of BT by endocytic vesicles via ATPase proton pumps. Step 3 occurs as the vesicular lumen becomes acidic, altering the structure, and translocating BT into the cytosol. Once in the cytosol, step 4 occurs when it is catalyzed. These steps all cause the inhibition of acetylcholine,2 an important player as an excitatory neurotransmitter located within neuromuscular junctions.4
Botulinum Toxin Preparations
Injecting BT can be done in an outpatient setting; when injected, BT takes approximately 2 to 3 days to take effect, and up to 2 weeks in some cases. The effects will last around 3 months depending on the patient, dosage, technique, and strength of the muscle. It is important that patients understand that BT injections are a temporary treatment.1
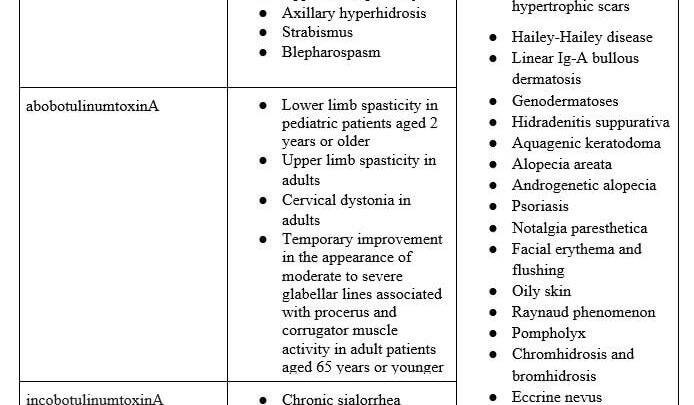
The most common preparations of BT — onabotulinumtoxinA, abobotulinumtoxinA, and incobotulinumtoxinA — can each be stored for up to 24 hours after they are reconstituted with 0.9% sodium chloride. While abobotulinumtoxinA is supplied in 300 or 500 unit vials,5 both onabotulinumtoxinA and incobotulinumtoxinA are distributed in 50, 100, or 200 units per vial.6,7
Treating Facial Lines With Botulinum Toxin
The most common areas to have lines and wrinkles include the forehead, glabella (frown lines), and around the eyes, all FDA approved indications. When BT is injected into the frontalis muscle, there is risk of temporary blepharoptosis and eyebrow ptosis; this risk decreases with improved technique from the injector.8
When treating frown lines, the targeted muscles are the corrugator supercilii and the procerus. Treating the corrugator supercilii causes the eyebrows to not be drawn medially, and treating the procerus prevents the eyebrows from being depressed.8
The lateral orbicularis oculi should be targeted to treat crows feet.8 Patients with dynamic wrinkles, meaning they are only present on muscle contraction, will benefit most from BT injections compared with those with wrinkles present at rest, who will require multiple treatments and maintenance for optimal results.9
Treating Primary Headaches With Botulinum Toxin
In Germany, there were case reports that injections of BT into muscles involving the neck and head to alleviate tension-type headaches.10 In 2010, BT was reported to be an effective agent in the management of chronic migraines via the Phase 3 Research Evaluative Migraine Prophylaxis Therapy trials.11
Botulinum toxin is injected in 5-unit increments in the glabellar, frontal, temporal, occipital, upper cervical, and trapezius areas.12 Extra BT can be injected into temporal, occipital, and trapezius areas to target specific locations of headaches.12
Evidence has shown that patients who receive BT for chronic migraine treatment earlier during their condition have increased likelihood for remission in the future.13
BT injections are often better tolerated by patients compared to oral migraine medications, and BT has also been tested in other headache conditions including trigeminal neuralgia, tension headaches, and headache from temporomandibular disorder.12
Treating Hyperhidrosis With Botulinum Toxin
Hyperhidrosis, an idiopathic condition which involves excess sweating, can be treated with BT. For treatment of hyperhidrosis, BT is administered intradermally in multiple injections. While treatment of axillary hyperhydrosis is an FDA-approved indication for BT, treatment of palmar or plantar hyperhidrosis are off-label uses. BT is a second-line treatment for patients with hyperhidrosis after topical treatments have been tried. BT blocks autonomic innervation of sweat glands; this effect lasts 3 to 6 months.14 In a study that followed up with patients treated with BT 5 years after treatment, patients had a significant improvement in quality-of-life outcomes.15
Treating Depression With Botulinum Toxin
The corrugator supercilii muscles as well as the procerus muscle in the glabellar area not only produce frown lines but they also influence the perception and expression of emotions. Major depressive disorder can benefit from BT injections, as it plays a role in conditions with an inclination for negative emotions, though this is an off-label use.16
Major depressive disorder is one of the leading causes of disability in the world; as of 2014, 264 million people had a depressive disorder.17 Patients who receive BT injections in the glabellar region for depression may see results for up to 3 months, which can be more convenient and less costly than other treatment options, such as antidepressant medication or psychotherapy, for depressive disorders.
Treating Urinary Incontinence With Botulinum Toxin
One of the first FDA-approved uses of BT was treating urinary incontinence. Botulism toxin can be used to cause chemical denervation to the detrusor muscle, responsible for contraction during urination, which pushes urine from the bladder into the urethra.18 Injections of BT into the detrusor muscle have been shown to decrease the frequency of urinary incontinence episodes.19 The benefits of BT may last up to 9 months, far longer than most BT injections. This treatment does however require a higher dosage when compared to other applications, around 200-300 units are necessary to see results.18
Treating Temporomandibular Dysfunction With Botulinum Toxin
Temporomandibular dysfunction (TMD) is characterized by pain in the jaw, crepitus, and movement dysfunction. Displacement of the articular disc in patients with TMD is a common finding. The masticatory muscles are composed of 4 pairs of muscles including the lateral pterygoid, medial pterygoid, temporalis, and masseter. The lateral pterygoid muscle provides horizontal movement to the condyle and attaches to the articular disc. Although there are many treatment options for TMD including oral appliances, medications, and arthroplasty, these options do not target specific masticatory muscles.
By specifically targeting the lateral pterygoid, clicking and crepitus of the articular disc can be reduced therefore reducing symptoms of TMD.20 Accessing the lateral pterygoid requires electromyography to aid in determining injection placement. Commonly, the masseter is treated with botox due to its ease of palpation, although this is considered an off-label use. Many providers do not have access to electromyography, this is currently a barrier to the use of this treatment for TMD. BT for TMD can last up to 6 months before requiring repeated treatment.20
Safety Considerations for Botulinum Toxin
Contraindications to BT include allergy, pregnancy, breastfeeding, body dysmorphic disorder, keloidal scarring, and neuromuscular disorders. The median lethal dose of BT is 1 to 3 nanograms per kilogram of body weight. Treatment for toxicity is antitoxin or vaccine. Although adverse effects are typically moderate, it is important for providers to be aware and provide education to patients receiving BT. Minor adverse effects include bruising, edema, and pain. Moderate adverse effects may include blepharoptosis or eyebrow ptosis lasting up to 3 months. Most adverse effects are dependent on the provider and their training. Allergic reactions are rare, but range from rashes to anaphylaxis.22
Key Takeaways on Clinical Uses for Botulinum Toxin
As use of BT continues to grow in popularity, it is important that providers are aware of all of its FDA-approved and off-label uses. Cosmetic applications can improve quality of life for many, and many other benefits of BT include treatment of hyperhidrosis, headache, TMD, urinary incontinence, depression, and more.
Olivia Jose BSN, RN, is affiliated with Brooks College of Health, University of North Florida in Jacksonville, Florida.
This article originally appeared on Clinical Advisor





